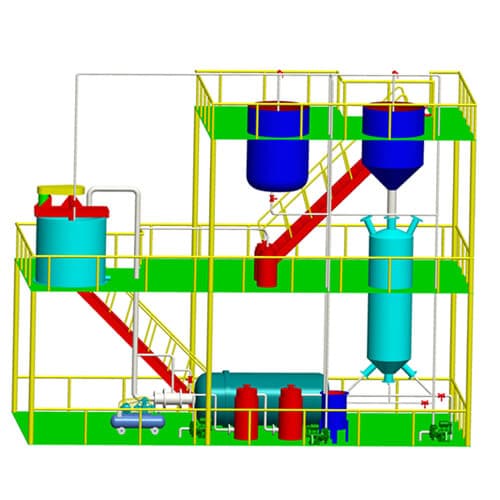
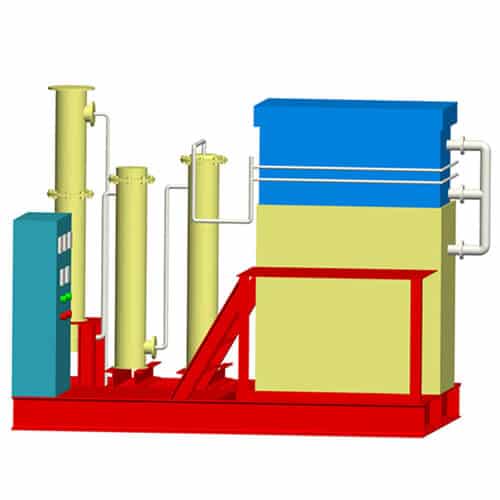
Desorption Electrolysis System
Gold electrolysis is the system which obtains gold mud from carbon by desorption and electrowinning. There are two process ways. One is in normal temperature and pressure desorption and electrolysis. Another is in high temperature and pressure desorption. This system consists of electrolysis equipment including desorption column and the electrodeposition tank.
Desorption Electrolysis System
Gold electrolysis is the system which obtains gold mud from carbon by desorption and electrowinning. There are two process ways. One is in normal temperature and pressure desorption and electrolysis. Another is in high temperature and pressure desorption. This system consists of electrolysis equipment including desorption column and the electrodeposition tank.
Description of gold desorption methods
1 Zadra desorption
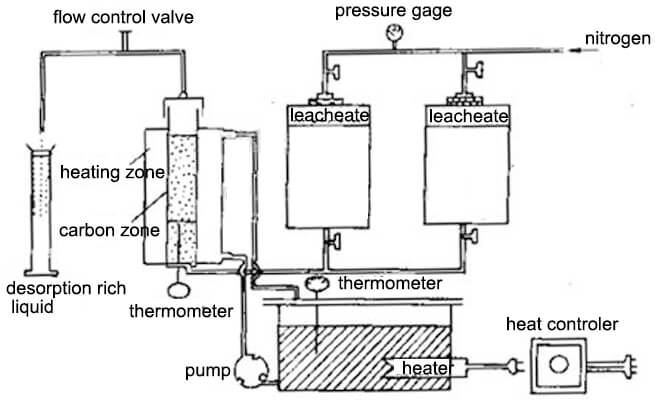
Although it has long been known that activated carbon is a good adsorbent for gold cyanide, the early method of recovering gold from gold-loaded carbon is high cost. In 1952 Zadra published a method for desorbing gold-loaded carbon with a hot sodium hydroxide-sodium cyanide mixed solution, namely Zadra desorption. The desorption method is to use a 1% sodium hydroxide solution and a 0.1% sodium cyanide mixed solution to heat to 90-95 ° C, the gold and silver on the gold loaded charcoal are desorbed during mixed solution pass through the gold-bearing carbon layer at a certain speed.
The result shows that the desorption rate of gold and silver is over 90% use 4-6h, in actual production, it usually takes 50 to 70 hours to achieve a high desorption rate. Zadra desorption method still greatly reduces the operating cost of gold mine heap leaching operations.
2 High temperature and high-pressure desorption
The high temperature and high-pressure desorption method is to install the gold-loaded carbon in a pressure vessel, and mix the solution with 0.4% to 1% NaOH and 0.1% NaCN, heat temperature to 130 to 160 ° C, under the pressure of 3.6 to 5.9 kg/cm2. Within 2 to 6 hours, the gold and silver desorption rates of gold-loaded carbon exceeded 90%. Compared with the Zara process, the high-pressure desorption method has a much shorter desorption time, and the desorption process also consumes fewer reagents than the Zadra process.
3 Alkaline ethanol solution desorption
The alkaline ethanol solution desorption method is to add 20% ethanol (or propanol) in a mixed solution of 1% NaOH and 0.1% to 0.2% NaCN, desorption process operates within 6-8 hours at 83° C temperature to finish. The volume of the adsorbent and the washing liquid is only 7 to 10 times the bed volume. The practice has shown that desorption with an alkaline ethanol solution can reduce the number of carbon regenerations. However, ethanol is flammable and explosive, and strict safety measures should be considered. Increasing the recovery of alcohols is critical to reducing the cost of the alcohol solution desorption process.
Normal temperature and pressure desorption at 78 ~ 80 ° C, 40% ~ 50% ethanol, NaOH 1% ~ 2%, NaCN 0.5% ~ 1.0%, 1-2h, then repeats the process with 30% to 35% ethanol solution. Distill the solution, the distilled steam passes through the desorbed carbon layer, ethanol vapor is cooled by the condenser into an alcohol solution and flows into the alcohol storage tank. pump lifts the distilled recovery alcohol to the desorption dome and flows through the carbon layer from top to bottom. Repeated distillation 3 to 4 times to complete a desorption cycle, generally 8 ~ 10h. The desorption rate is greater than 98% and the recovery of ethanol is greater than 90%. The concentration of the rich liquid gold obtained by this method can reach 5-8 g ∕L (the gold grade of the char). Therefore, the current efficiency during electrolysis is also higher than other methods. Since the alcohol vapor repeatedly passes through the carbon layer during desorption, it is beneficial to restore the activity of the carbon, and the desorbed carbon can be reused without high-temperature regeneration.