In the mining and chemical industries, barite (chemical formula BaSO₄) is an important non-metallic mineral with wide applications. It plays an irreplaceable role in industries such as oil drilling, barium chemicals, medical imaging, and radiation protection. With the depletion of high-grade resources and the steady growth of downstream demand, barite, which has been overlooked in tailings, is becoming a considerable secondary resource.
The following section introduces the basic characteristics of barite, its global distribution, why it is commonly found in lead-zinc tailings, and an effective recycling process, and presents its experimental results in a simple yet rigorous manner.
Barite Resource Characteristics and Global Industrial Landscape
As a strategic non-metallic mineral, barite holds significant importance in industrial development due to its unique physicochemical properties and extensive applications. Its high density, acid-alkali resistance, and radiation shielding capabilities create irreplaceable application scenarios, particularly its pivotal role in oil and gas drilling, which drives stable global demand growth. Concurrently, as high-grade primary resources diminish, the industry is increasingly prioritizing the recovery and utilization of by-products, such as lead-zinc tailings. This trend necessitates a systematic understanding of barite's fundamental characteristics and applications.
Globally, barite exhibits pronounced regional concentration. Resource fluctuations in major producing nations like China directly influence international market supply and demand dynamics. Against the backdrop of increasingly complex resource development, understanding barite's resource endowment characteristics and regional distribution is crucial for formulating rational resource development policies and optimizing comprehensive tailings utilization technologies. The following sections will respectively elaborate on barite's fundamental properties and application value, as well as the current status and development trends of resources in major global production areas.
Basic Characteristics and Primary Applications of Barite
Barite (BaSO₄) exhibits the following typical properties
- High density: Approximately 4.2–9 g/cm³, significantly higher than common gangue minerals;
- Chemical inertness: Insoluble in water and most acids/bases;
- Non-toxic, with effective absorption of X-rays and gamma rays.
These characteristics determine its primary applications
- Drilling mud weighting agent (oil and gas exploration) — its largest application;
- Barium chemical feedstock (e.g., for producing barium salts);
- Radiation shielding material and medical imaging contrast agent;
- Filler for plastics, rubber, coatings, etc.

Major Production Areas and Resource Status
Barite resources are widely distributed globally, with primary producing countries including China, India, Morocco, the United States, Russia, and Mexico. China has long held a leading position in both barite reserves and production. However, the gradual depletion of high-grade deposits has prompted the industry to explore the comprehensive utilization of medium- to low-grade ores and tailings resources.
Why Do Lead-zinc Tailings Contain Barite?
In many lead-zinc deposits, barite commonly occurs alongside sulfide ores (such as galena and sphalerite) and carbonate gangue minerals (such as dolomite). Lead-zinc beneficiation processes primarily recover metal sulfides through flotation. Barite, being non-metallic with weak natural hydrophobicity or tightly associated with gangue minerals, is often excluded from the main product and discharged with the tailings. Consequently, tailings ponds frequently contain economically valuable barite deposits. However, without recovery and processing, this represents a significant waste of resources.
Process for Recovering Barite from Tailings
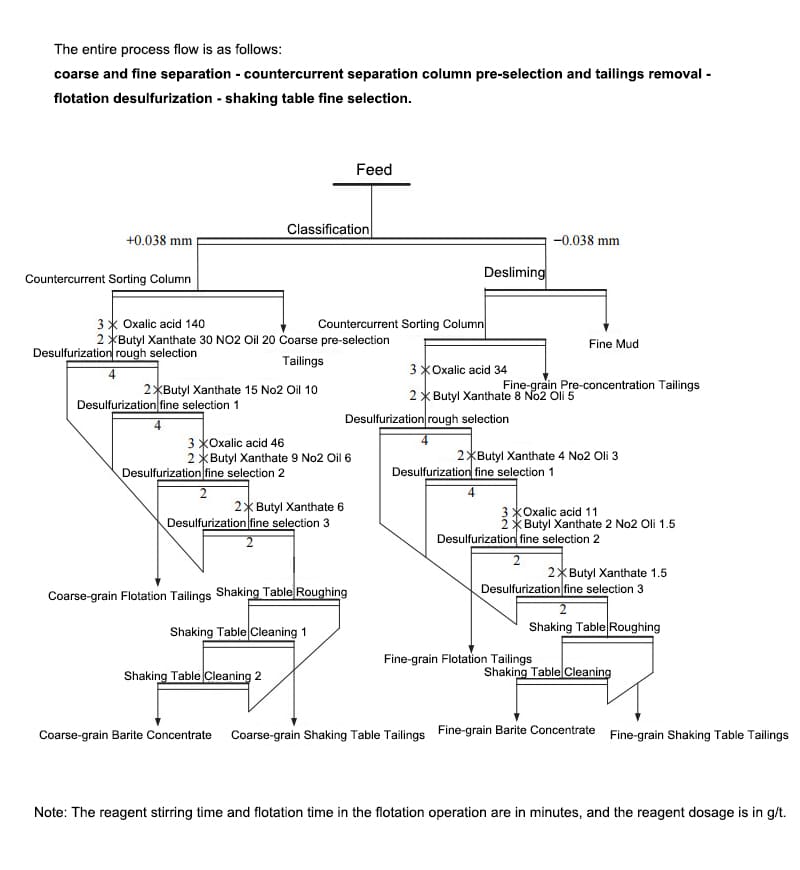
The recovery process for barium carbonate from tailings primarily relies on physical property differences (density variation) and chemical de-impurity treatment to produce high-grade barium carbonate concentrate. A typical and effective workflow can be summarized as follows:
Coarse-fine classification
Separate tailings into coarse and fine particles based on grain size for targeted processing and improved separation efficiency.
Gravity Pre-concentration (Streak Removal)
Utilize gravity separation equipment (e.g., counter-current separation columns, spiral chutes, centrifugal concentrators) to first remove light gangue (dolomite, quartz, etc.), concentrating barite and heavy minerals like pyrite.
Floating Desulfurization
Due to pyrite's density proximity to barite, both minerals often co-concentrate post-gravity separation. Reverse flotation or desulfurization flotation is required to remove sulfide impurities like pyrite.
Shaking Table / Concentration
Further purification of desulfurized concentrates using shaking tables or similar concentration equipment yields high-grade barite concentrate.
This approach balances practical production requirements: a compact process flow, compatibility of reagent recycling water with the main process, and high product grade.
Recovering Barite from Lead-zinc Tailings Example
Experimental Validation and Key Data
| Table 1: Results of Classified Cleaning by Shaking | ||||
| Particle Size/mm | Product Name | Working Yield /% | BaSO4 Grade /% | BaSO4 Work Recovery Rate /% |
| +0.038 | Coarse-grained Barite Concentrate | 55.02 | 92.76 | 73.72 |
| Coarse-grained Tailings | 44.98 | 40.45 | 26.28 | |
| Feed | 100.00 | 69.23 | 100.00 | |
| -0.038 | Fine-grained Barite Concentrate | 71.77 | 92.21 | 81.34 |
| Fine-grained Tailings | 28.23 | 53.76 | 18.66 | |
| Feed | 100.00 | 81.35 | 100.00 | |
| Table 2: Results of “Coarse and Fine Fractions Classification - Pre-discarding by Reflux Classifier - Flotation Desulfurization - Shaking Table Cleaning” Process | |||||||
| Product Name | Working Yield /% | Grade /% | Recovery Rate /% | ||||
| BaSO4 | Fe | MgO | BaSO4 | Fe | MgO | ||
| Coarse-grain Flotation Tailings | 8.95 | 22.18 | 19.34 | 5.68 | 5.51 | 35.02 | 5.64 |
| Coarse-grain Barite Concentrate | 12.09 | 92.76 | 0.20 | 0.14 | 31.13 | 0.48 | 0.19 |
| Coarse-grain Shaking Table Tailings | 9.89 | 40.45 | 1.90 | 10.83 | 11.10 | 3.81 | 11.89 |
| Coarse-grain Pre-concentration Tailings | 221.36 | 5.15 | 3.60 | 15.03 | 3.05 | 15.56 | 35.64 |
| Fine-grain Flotation Tailings | 1.35 | 22.41 | 28.58 | 3.14 | 0.84 | 7.81 | 0.47 |
| Fine-grain Barite Concentrate | 4.43 | 92.21 | 0.50 | 0.20 | 11.34 | 0.45 | 0.10 |
| Fine-grain Shaking Table Tailings | 1.74 | 53.77 | 2.12 | 7.10 | 2.60 | 0.75 | 1.37 |
| Fine-grain Pre-concentration Tailings | 19.69 | 24.25 | 3.35 | 13.03 | 13.25 | 13.35 | 28.48 |
| Fine Slime | 20.50 | 37.22 | 5.49 | 7.12 | 21.18 | 22.77 | 16.20 |
| Total Barite Concentrate | 16.52 | 92.61 | 0.28 | 0.16 | 42.47 | 0.94 | 0.29 |
| Feed | 100.00 | 36.03 | 4.94 | 9.01 | 100.00 | 100.00 | 100.00 |
Based on recent experimental research, the following key points can be derived from test results on typical lead-zinc flotation tailings:
Process Flow
Coarse-fine classification → Counter-current column pre-selection and tailings rejection → Flotation desulfurization → Shaking table final concentration. This process centers on gravity separation, utilizing flotation to remove pyrite (with a density close to barite) and shaking tables for final purification.
Representative Experimental Results
The final barite concentrate achieved a BaSO₄ grade of approximately 92.61%, with an overall recovery rate of about 42.47% (based on the test sample mass). This demonstrates a balance between feasibility and economics in recovering barite from tailings.
The counter-current separation column effectively improved concentrate grade and reduced downstream load during coarse-fine pre-selection tailings rejection. In the flotation stage, oxalic acid as an activator and butyl xanthate as a collector efficiently removed pyrite, reducing Fe content below 1%. Final shaking table concentration further elevated the BaSO₄ grade to over 92%.
Summarize
Tailings are not “waste” but potential secondary mines. Recovering barite from lead-zinc tailings requires selecting either a “gravity-flotation combined” process or a single flotation process based on mineral distribution characteristics. Through a rational combination of physical separation and chemical de-impurification, the overlooked barite resources in lead-zinc tailings can be transformed into high-grade products. This approach not only enhances resource utilization but also creates new revenue streams for enterprises. It is recommended to conduct laboratory selectivity tests first, followed by scaling up to an industrial scale.
